Vaccines
Table of Contents
Background
Through ongoing work with immunization information systems in multiple LMICs, the OpenLMIS team is seeing a consistency in requests coming from EPI programs and their partners regarding the functionality and reporting needed to manage vaccine logistics and program data.
These requests include a common need across countries for flexible vaccine data collection tools, a consolidated view of vaccine service delivery and logistics data, cold chain inventory management, integration with/replacement of the DVD-MT, and vaccine stock management.
The bulk of the work on new vaccine-specific functionality for OpenLMIS falls into two areas: (i) front-end data collection flexibility and configurability for different supply chain designs and associated workflow variations (i.e., push and pull distribution models); and (ii) improved reporting and analytics.
See OpenLMIS Global Vaccine Functionality
Scope
The scope of vaccine module is to manage vaccine need calculation, requisition/push, distribution, stock management, cold chain, and reporting. Draft list of module epics:
- Forecasting, Estimation & Demand Planning
- Re-supplying of Health Facilities and sites
- Stock Management
- Cold Chain Equipment Reporting & Management
- Configuration
- Reports and key DISC KPIs
OpenLMIS Acceleration Brief
As a supplement to the initial Bill & Melinda Gates Foundation grant, VillageReach was awarded additional funding to accelerate and complete the re-architecture of OpenLMIS and release the version 3 series. The development of the vaccine module was also included in the supplemental award.
Additional information from Acceleration Brief on areas of focus for the vaccine module:
(i) Front-end data collection flexibility and configurability for different vaccine supply chain designs and associated workflow variations (i.e., push and pull distribution models)
(ii) Vaccine reporting and analytics
(iii) Vaccine demand planning (ideal stock amount, population data management, replenishment calculations)
(iv) Vaccine stock management, including accounts for doses vs. vials, and robust tracking of expiry dates, batch and VVM status (optionally via bar coding)
(v) Vaccine cold chain equipment management and integration
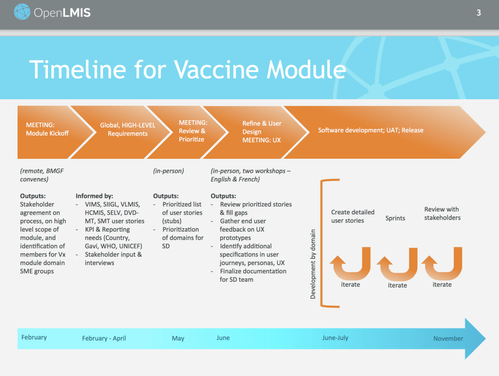
Timeline
See the Living Product Roadmap for details on the software development timelines.
Features
After the initial workshop and prioritization, the current initial MVP scope is outlined here.
Related content
OpenLMIS: the global initiative for powerful LMIS software